What is the PSA test?
Prostate-specific antigen, or PSA, is a protein produced by normal, as well as malignant, cells of the prostate gland. The PSA test measures the level of PSA in the blood. For this test, a blood sample is sent to a laboratory for analysis. The results are usually reported as nanograms of PSA per milliliter (ng/mL) of blood.
The blood level of PSA is often elevated in people with prostate cancer, and the PSA test was originally approved by the FDA in 1986 to monitor the progression of prostate cancer in men who had already been diagnosed with the disease. In 1994, FDA approved the PSA test to be used in conjunction with a digital rectal exam (DRE) to aid in the detection of prostate cancer in men 50 years and older. Until about 2008, many doctors and professional organizations had encouraged yearly PSA screening for prostate cancer beginning at age 50.
PSA testing (along with a DRE) is also often used by health care providers for individuals who report prostate symptoms to help determine the nature of the problem.
In addition to prostate cancer, several benign (not cancerous) conditions can cause a person’s PSA level to rise, particularly prostatitis (inflammation of the prostate) and benign prostatic hyperplasia (BPH) (enlargement of the prostate). There is no evidence that either condition leads to prostate cancer, but someone can have one or both of these conditions and develop prostate cancer as well.
Is the PSA test recommended for prostate cancer screening?
Beginning around 2008, as more was learned about both the benefits and harms of prostate cancer screening, a number of professional medical organizations began to caution against routine population screening with the PSA test. Most organizations recommend that individuals who are considering PSA screening first discuss the risks and benefits with their doctors.
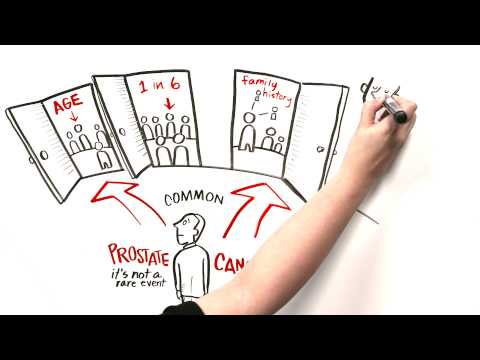
Some organizations do recommend that men who are at higher risk of prostate cancer begin PSA screening at age 40 or 45. These include Black men, men with germline variants in BRCA2 (and to a lesser extent, in BRCA1), and men whose father or brother had prostate cancer.
In 2018, the United States Preventive Serves Task Force (USPSTF) updated its recommendation statement for prostate cancer screening Exit Disclaimer from a “D” (not recommended) to a “C” (selectively offering PSA-based screening based on professional judgment and patient preferences) in men ages 55 to 69. (The USPSTF continues to recommend against PSA screening for men 70 years and older.) The updated recommendation, which applies to the general population as well as those at increased risk due to race/ethnicity or family history, is as follows:
- For individuals ages 55 to 69 years, the decision to undergo periodic PSA-based screening for prostate cancer should be an individual one. Before making the decision, a person should discuss the potential benefits and harms of screening with their clinician and consider these in the context of their own values and preferences.
- PSA-based screening for prostate cancer is not recommended for individuals 70 years and older.
Currently, Medicare provides coverage for an annual PSA test for all Medicare-eligible individuals ages 50 and older. Many private insurers cover PSA screening as well.
What is a normal PSA test result?
There is no specific normal or abnormal level of PSA in the blood. In the past, PSA levels of 4.0 ng/mL and lower were considered normal. However, some individuals with PSA levels below 4.0 ng/mL have prostate cancer and many with higher PSA levels between 4 and 10 ng/mL do not have prostate cancer.
In addition, various factors can cause someone’s PSA level to fluctuate. For example, the PSA level tends to increase with age, prostate gland size, and inflammation or infection. A recent prostate biopsy will also increase the PSA level, as can ejaculation or vigorous exercise (such as cycling) in the 2 days before testing. Conversely, some drugs—including finasteride and dutasteride, which are used to treat BPH—lower the PSA level.
In general, however, the higher a man’s PSA level, the more likely it is that he has prostate cancer.
What is done if a screening test shows an elevated PSA level?
If someone who has no symptoms of prostate cancer chooses to undergo prostate cancer screening and is found to have an elevated PSA level, the doctor may recommend another PSA test to confirm the original finding. If the PSA level is still high, the doctor may recommend that the person continue with PSA tests and digital rectal exams (DREs) at regular intervals to watch for any changes over time (also called observation or watchful waiting).
If the PSA level continues to rise or a suspicious lump is detected during a DRE, the doctor may recommend additional tests to determine the nature of the problem. These may include imaging tests, such as magnetic resonance imaging (MRI) or high-resolution micro-ultrasound.
Alternatively, the doctor may recommend a prostate biopsy. During this procedure, multiple samples of prostate tissue are collected by inserting hollow needles into the prostate and then withdrawing them. The biopsy needle may be inserted through the wall of the rectum (transrectal biopsy) or through the perineum (transperineal biopsy). A pathologist then examines the collected tissue under a microscope. Although both biopsy techniques are guided by ultrasound imaging so the doctor can view the prostate during the biopsy procedure, ultrasound cannot be used alone to diagnose prostate cancer. An MRI-guided biopsy may be performed for patients with suspicious areas seen on MRI.
In the past, men with elevated PSA levels and no other symptoms were sometimes prescribed antibiotics to see if an infection might be causing the PSA increase. However, according to the American Urological Association, there is no evidence to support the use of antibiotics to reduce PSA levels in men who are not experiencing other symptoms.
What are some of the limitations and potential harms of the PSA test for prostate cancer screening?
Detecting prostate cancer early may not reduce the chance of dying from prostate cancer. When used in screening, the PSA test can help detect small tumors. Having a small tumor found and treated may not, however, reduce the chance of dying from prostate cancer. That is because many tumors found through PSA testing grow so slowly that they are unlikely to be life threatening. Detecting such tumors is called “overdiagnosis,” and treating them is called “overtreatment.”
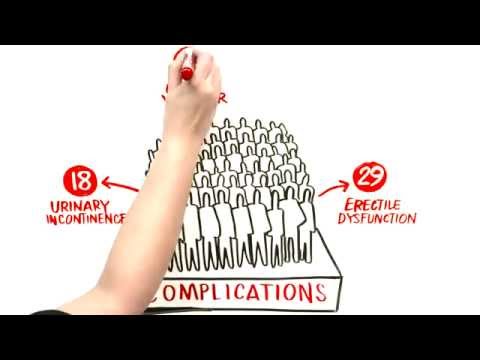
Overtreatment exposes a person unnecessarily to the potential complications associated with prostate surgery and radiation therapy. These include urinary (e.g., urinary incontinence, or leaking of urine following surgery and increased frequency and urgency of urination following radiation), gastrointestinal (e.g., loose stools or, less commonly, rectal bleeding following radiation), and sexual side effects (loss of erections or decreased erections following both surgery and radiation).
In addition, finding cancer early may not help someone who has a fast-growing or aggressive prostate tumor that may have spread to other parts of the body before being detected.
The PSA test may give false-positive results. A false-positive test result occurs when the PSA level is elevated but no cancer is actually present. A false-positive test result may create anxiety and lead to additional medical procedures, such as a prostate biopsy, that can be harmful. Possible side effects of biopsies include serious infections, pain, and bleeding.
False-positive test results are common with PSA screening; only about 25% of people who have a prostate biopsy due to an elevated PSA level are found to have prostate cancer when a biopsy is done.
What have randomized trials of prostate cancer screening found?
Several large, randomized trials of prostate cancer screening have been carried out. One of the largest is the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which NCI conducted to determine whether certain screening tests can help reduce the numbers of deaths from several common cancers. In the prostate portion of the trial, the PSA test and digital rectal exam were evaluated for their ability to decrease a man’s chances of dying from prostate cancer.
The PLCO investigators found that men who underwent annual prostate cancer screening had a higher incidence of prostate cancer than men in the control group but had about the same rate of deaths from the disease. Overall, the results suggest that many men were treated for prostate cancers that would not have been detected in their lifetime without screening. Consequently, these men were exposed unnecessarily to the potential harms of treatment.
A second large trial, the European Randomized Study of Screening for Prostate Cancer (ERSPC), compared prostate cancer deaths in men randomly assigned to PSA-based screening or no screening. As in the PLCO, men in ERSPC who were screened for prostate cancer had a higher incidence of the disease than control men. In contrast to the PLCO, however, men who were screened had a lower rate of death from prostate cancer.
A subsequent analysis of data from the PLCO used a statistical model to account for the fact that some men in the PLCO trial who were assigned to the control group had nevertheless undergone PSA screening. This analysis suggested that the level of benefit in the PLCO and ERSPC trials was similar and that both trials showed some reduction in prostate cancer death in association with prostate cancer screening.
Such statistical modeling studies have important limitations and rely on unverified assumptions that can render their findings questionable (or more suitable for further study than to serve as a basis for screening guidelines). More important, the model could not provide an assessment of the balance of benefits versus harms from screening.
The third and largest trial, the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP), conducted in the United Kingdom, compared prostate cancer mortality among men whose primary care practices were randomly assigned to offer their patients a single PSA screening test or to provide usual care in which screening was not offered. After a median follow-up of 10 years, more low-risk prostate cancers were detected in the single PSA test group than in the usual (unscreened) care group (even though only about a third of men in the screening group actually had the PSA test), but there was no difference in prostate cancer mortality.
A systematic review and meta-analysis of all randomized controlled trials comparing PSA screening with usual care in men without a diagnosis of prostate cancer concluded that PSA screening for prostate cancer leads to a small reduction in prostate cancer mortality over 10 years but does not affect overall mortality.
The United States Preventive Services Task Force has estimated that, for every 1,000 men ages 55 to 69 years who are screened for 13 years:
- About 1.3 deaths from prostate cancer would be avoided (or 1 death avoided per 769 men screened). [However, based on 3 additional years of follow-up in the ERSPC trial, about 1.8 deaths from prostate cancer would be avoided per every 1,000 men screened, or 1 death in 570 men screened].
- 3 men would avoid developing metastatic cancer
- 5 men would die from prostate cancer despite screening, diagnosis, and treatment
- 240 men would have a positive PSA test result, many of whom would have a biopsy that shows that the result was a false-positive; some men who had a biopsy would experience at least moderately bothersome symptoms (pain, bleeding, or infection) from the procedure (and 2 would be hospitalized).
- 100 men would be diagnosed with prostate cancer. Of those, 80 would be treated (either immediately or after a period of active surveillance) with surgery or radiation. Many of these men would have a serious complication from treatment, with 50 experiencing sexual dysfunction and 15 experiencing urinary incontinence.
- 200 men would die of causes other than prostate cancer
How is the PSA test used in men who have been treated for prostate cancer?
The PSA test is used to monitor men after surgery or radiation therapy for prostate cancer to see if their cancer has recurred (come back). If a man’s PSA level begins to rise after prostate cancer treatment, it may be the first sign of a recurrence. Such a “biochemical relapse” typically appears months or years before the recurrence causes symptoms.
However, a single elevated PSA measurement in someone who has a history of prostate cancer does not always mean that the cancer has come back. Someone who has been treated for prostate cancer should discuss an elevated PSA level with their doctor. The doctor may recommend repeating the PSA test or performing other tests to check for evidence of a recurrence. The doctor may look for a trend of rising PSA level over time rather than a single elevated PSA level.
A rising trend in PSA level over time in combination with other findings, such as an abnormal result on imaging tests, may lead the doctor to recommend further cancer treatment.
How are researchers trying to improve the PSA test?
Scientists are investigating ways to improve the PSA test to give doctors the ability to better distinguish cancerous from benign conditions and slow-growing cancers from fast-growing, potentially lethal cancers. And other potential biomarkers of prostate cancer are being investigated. None of these tests has been proven to decrease the risk of death from prostate cancer. Some of the methods being studied include
- Free versus total PSA. The amount of PSA in the blood that is “free” (not bound to other proteins) divided by the total amount of PSA (free plus bound) is denoted as the proportion of free PSA. Some evidence suggests that a lower proportion of free PSA may be associated with more aggressive cancer.
- PSA density. The blood level of PSA divided by the volume of the prostate gland. Some evidence suggests that this measure may be more accurate at detecting prostate cancer than the standard PSA test.
- PSA velocity and PSA doubling time. PSA velocity is the rate of change in a man’s PSA level over time, expressed as ng/mL per year. PSA doubling time is the period of time over which a man’s PSA level doubles. These measures are most useful in men with a biochemical recurrence following surgery or radiation therapy.
- Pro-PSA. Pro-PSA refers to several different inactive precursors of PSA. There is some evidence that pro-PSA is more strongly associated with prostate cancer than with BPH. One blood test combines the measurement of a form of pro-PSA called [-2]proPSA with measurements of PSA and free PSA into a mathematical formula called the Prostate Health Index. The resulting “phi score” calculated from this formula can be used to help a man with a PSA level between 4 and 10 ng/mL decide whether he should have a biopsy.
- IsoPSA. PSA exists in different structural forms (called isoforms) in the blood. The IsoPSA test, which measures the entire spectrum of PSA isoforms rather than the concentration of PSA in the blood, may be better than traditional PSA testing for identifying men with an increased risk for developing high-grade prostate cancer who should undergo biopsy (11).
- 4Kscore Test. The 4Kscore test takes into account four different prostate-specific biomarkers, namely, total PSA, free PSA, intact PSA, and human kallikrein 2, as well as the patient’s age, prior biopsy history, and DRE status to assess the risk of aggressive prostate cancer in someone with an abnormal screening result.
- Urinary biomarkers. Prostate cancer antigen 3 (PCA3) mRNA and the TMPRSS2-ERG gene fusion are biomarkers that are tested in a urine sample. They have increased specificity for prostate cancer compared with PSA testing alone, but do not appear to preferentially identify clinically significant disease. The use of these two biomarkers in combination can help reduce the number of (unnecessary) biopsies.